Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
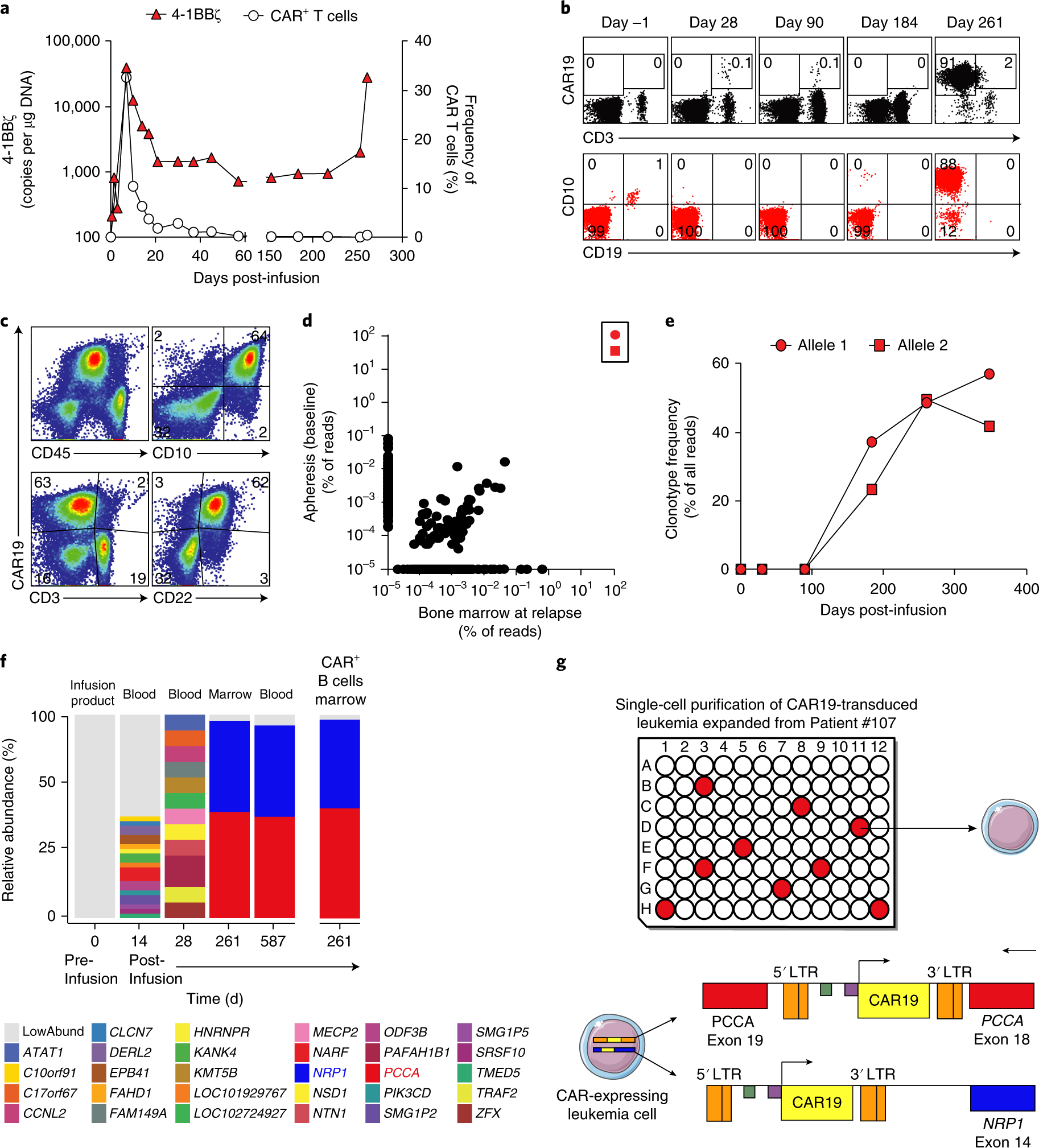
Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell | Nature Medicine
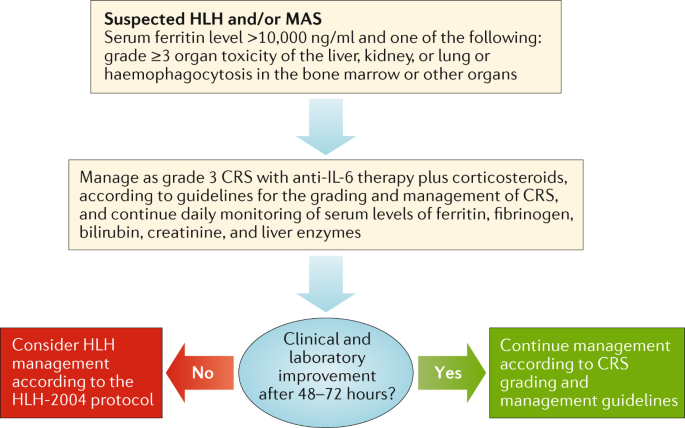
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology
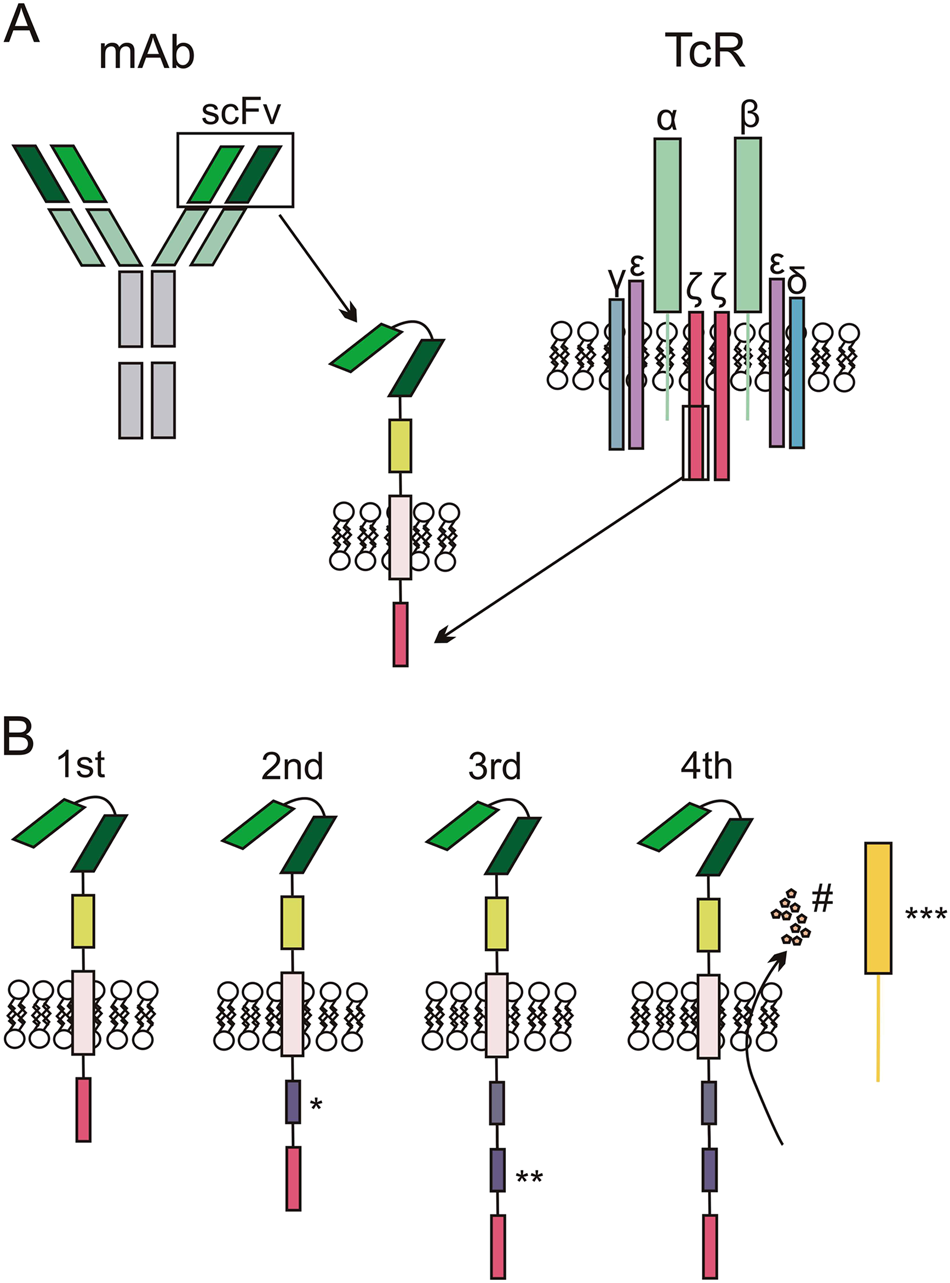
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA

A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy - Frazer A. Tessema, Jonathan J. Darrow, 2017
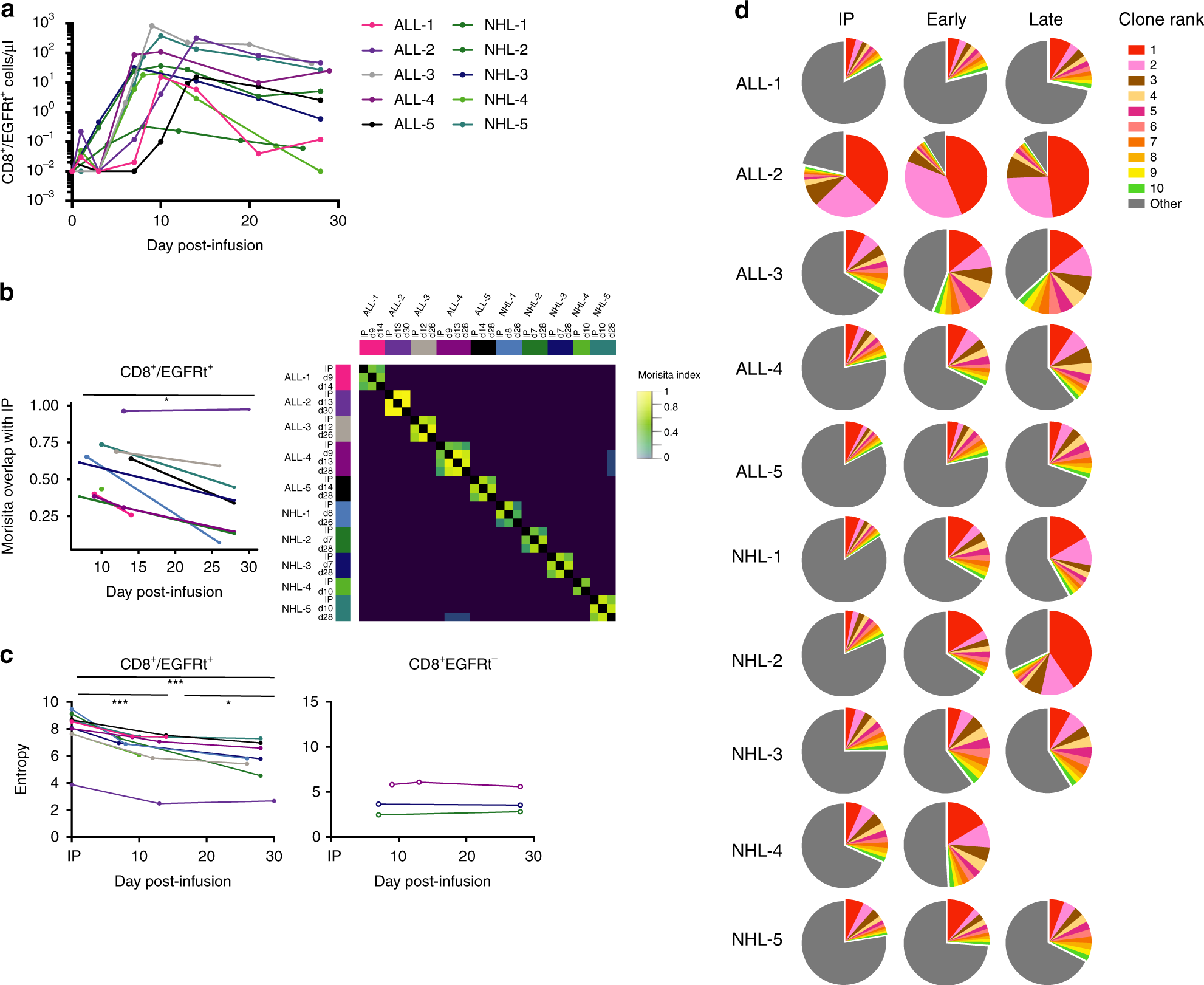
Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy | Nature Communications

Manufacturing News of Note—Novartis to have Cell Therapies make Kymriah in Australia; Piramal Pharma expands API plant in Canada | Fierce Pharma

ASCO: Gilead's Kite soars over Novartis' CAR-T turf with Tecartus win in type of leukemia | Fierce Pharma
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

Can CAR-T and gene therapy cures really sustain biopharma? Not for all, analyst says | Fierce Pharma
A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy - Frazer A. Tessema, Jonathan J. Darrow, 2017